Have additional questions?
Connect with CSL Behring Medical Affairs to find additional information and ask questions.


In the clinical studies, the most common adverse reactions, observed in >5% of study subjects, were headache, fatigue, nausea, chills, vomiting, back pain, pain, elevated body temperature, abdominal pain, diarrhea, cough, stomach discomfort, chest pain, joint swelling/effusion, influenza-like illness, pharyngolaryngeal pain, urticaria, and dizziness. Serious adverse reactions were hypersensitivity, chills, fatigue, dizziness, and increased body temperature.
Connect with CSL Behring Medical Affairs to find additional information and ask questions.


PATH, one of two clinical trials,† was the largest ever Ig study in CIDP, evaluating 207 patients
In the clinical studies, the most common reactions, observed in >5% of subjects, were headache, asthenia, hypertension, nausea, pain in extremity, hemolysis, influenza-like illness, leukopenia, and rash. A serious adverse reaction was hemolysis.
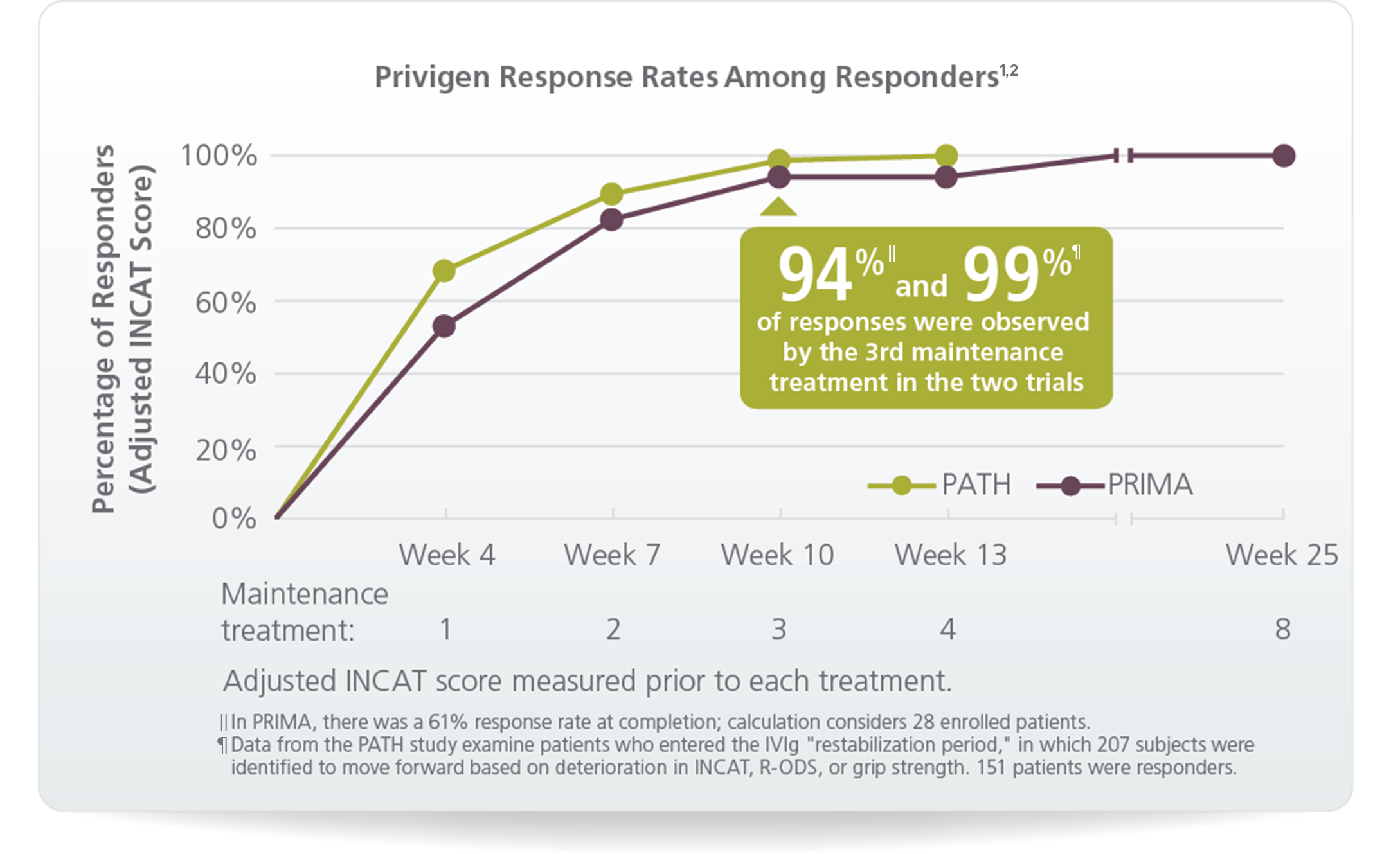

In PRIMA:
In PATH:
Connect with CSL Behring Medical Affairs to find additional information and ask questions.


* A prospective, open-label, single-arm study assessed the efficacy and safety of PRIVIGEN in 57 subjects with chronic ITP and a platelet count of ≤20 x 10^9/L. The Privigen dose was 1 g/kg given on 2 consecutive days (2 g/kg total dose). The primary endpoint was the percentage of subjects with an increase in platelet counts to ≥50 x 10^9/L within 7 days of the first infusion.
Connect with CSL Behring Medical Affairs to find additional information and ask questions.
Use the guide or dosing calculator to determine the right amount of Privigen for your patients
Find out what proline is and why it's in Privigen
References: 1. Data on file. Available from CSL Behring as DOF PVG-006. 2. National Institutes of Health: ClinicalTrials.gov. Safety and efficacy of intravenous immunoglobulin IgPro10 in patients with primary immunodeficiencies. https://clinicaltrials.gov/ct2/show/results/NCT00322556. Accessed January 22, 2025. 3. Stein MR, Nelson RP, Church JA, et al. Safety and efficacy of Privigen, a novel 10% liquid immunoglobulin preparation for intravenous use, in patients with primary immunodeficiencies. J Clin Immunol. 2009;29(1):137-144.
References: 1. Robak T, Salama A, Kovaleva L, et al. Efficacy and safety of Privigen®, a novel liquid intravenous immunoglobulin formulation in adolescent and adult patients with chronic immune thrombocytopenic purpura. Hematology. 2009;14(4):227-236.
References: 1. Data on file. Available from CSL Behring as DOF PVG-003. 2. Leger, JM et al. on behalf of the PRIMA study investigators. Efficacy and safety of Privigen® in patients with chronic inflammatory demyelinating polyneuropathy: results of a prospective, single-arm, open-label Phase III study (the PRIMA study ). Journal of the Peripheral Nervous System. 2013;18(3):130-140.