What is Privigen?
Privigen is an intravenous Ig therapy that contains antibodies—essential proteins in your immune system that identify and destroy bacteria and viruses that cause disease. The primary antibody found in Privigen, immunoglobulin G, is the main type of antibody made by your immune system.
Your doctor may refer to your Privigen treatment as "Ig," "intravenous Ig," or "IVIg."
What is chronic inflammatory demyelinating polyneuropathy (CIDP)?
CIDP is a rare disorder of the nervous system in which the protective outer layer of your nerves, called myelin, is attacked by the immune system.
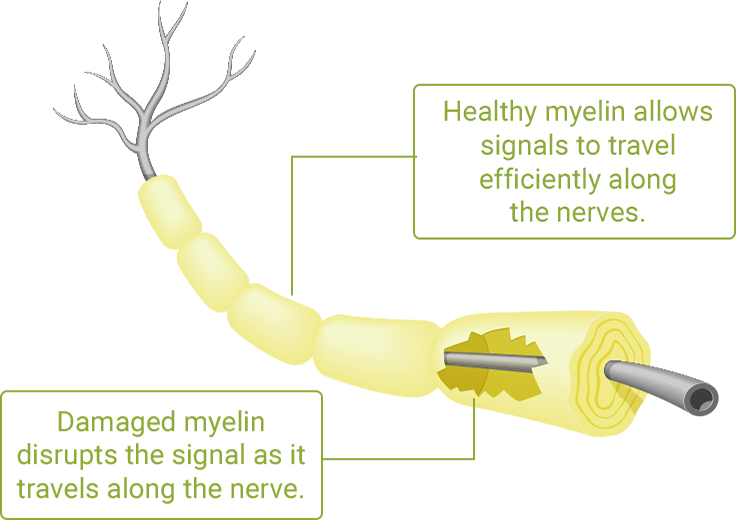
Over time, the myelin damage may cause gradual weakness and a loss of feeling in your arms and legs—and loss of movement (motor function). If CIDP is not treated, it can cause permanent nerve damage.

CIDP symptoms may include:
- Tingling or numbness beginning in the toes and fingers
- Weakness of the arms or legs
- Loss of reflexes
- Fatigue
How does Privigen work in CIDP?
Privigen helps improve motor function in adults with CIDP. It provides antibodies that may help block your immune system from attacking the myelin. The exact way that Privigen works is not completely understood.
Privigen is proven safe & effective
Study ResultsReceiving Privigen therapy
Infusion Process
